Introduction to pLAS2w, pLAS3w, and AS4.1w
series vectors
In the past two years, RNAi Core has conducted several R&D
projects to develop new lentiviral transfer vectors for facilitating biomedical
researchers in Taiwan. Some background information and experimental data for
the vectors, for instance virus titer, promoter strength, tightness and
responsibility of the tet-inducible system, are provided for your information.
The information is described as follows:
A.
All-in-one inducible
lentiviral transfer vector
The tet-inducible
vectors launched previously by the RNAi Core were composed of a two- vector
system as shown in the following Figure.
In this system, advanced On (aOn) or advanced Off (aOff)
transactivator is constitutively expressed from pAS3w.aOn.Pbsd or
pAS3w.aOff.Pbsd vector, respectively. The aOn then activates transcription of the
TRE-based promoter in the presence of doxycycline, an inducer of tet operator;
whereas aOff activates transcription of the TRE-based promoter in the absence of
doxycycline. On the other hand, doxycycline binds to aOff transactivator resulting
in loss of promoter binding activity, thereby inactivates transcription of
TRE-based promoter.
The all-in-one
vectors are the products that aOn transactivator was introduced into the
downstream of PAC or bsd gene on
pAS4.1w series plasmids by fusing it with a F2A (a small protease from picornavirus with cis
cleavage activity) sequence to link PAC or Bsd ORF together. The aOn ORF was designed to be the same
reading frame as PAC or Bsd;
as a result, this fusion protein will be post-translationally cleaved by F2A protease into aOn and PAC or Bsd
functional proteins.
The map of the
all-in-one vectors is showed as followed:
These vectors can be directly used to prepare VSV-G pseduotyped
lentivirus by following the standard protocol. Virus-transduced cells followed
by drug selection are readily to get stable cells with doxycycline inducible capability.
To test the tightness and inducibility of the all-in-one vector, ORF
of annexin A2 was introduced into the downstream of TRE promoter on
pAS4.1w.Ppuro-aOn vector to generate pAnxA2-aOn vector. As a control, annexin
A2 was also introduced into parental pAS4.1w.Ppuro, a vector without aOn ORF (pAS4.1w.AnxA2). VSV-G
pseduotyped lentiviruses prepared from these vectors were then transduced into
293T cells, a cell line expressing basal level of annexin A2. After selection
with 2 ug/ml of puromycin for one week, the expression of annexin A2 was
examined in the presence or absence of doxycycline (1 ug/ml) for five days (You
may titrate the amounts of doxycycline to get optimal expression of the inserted
gene ). As shown in the following figure,
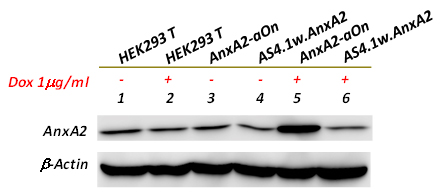
Annexin A2 was significantly induced after adding doxycycline
(lane 5) compared to those cells that without adding doxycycline (lane 3). In
addition, pAnxA2-aOn-tranduced cells in absence of doxycycline did not exhibit
obvious leakage compared to that of naive 293T cells or
pAS4.1w.AnxA2-transduced cells (compared lane 3 to lanes 1, 2, 4 or 6). All together, the results demonstrate that
the gene expression controlled by pAS4.1w.Ppuro-aOn
vector is very tight and the vector also possesses good responsibility to doxycycline
induction.
B.
New generation of
lentiviral transfer vectors with the capability of higher virus titer production
From historical point of view, the new vectors
described here are the third generation of vectors developed by the RNAi Core.
The first generation vectors was the pLKO_ AS2 series vectors. In these
vectors, a bicistronic RNA driven by CMV promoter was designed to translate
cDNA/ORF from 5’ end cap
and selection marker from IRES (EMCV). In
the second generation vectors, pLKO_AS3w series vectors, CMV promoter was
replaced by CAG promoter (a strong promoter composed of CMV enhancer region and
mouse beta-actin promoter). Additionally,
a WPRE sequence was also introduced into the downstream of CAG promoter to
promote the transcription of the promoter.
After releasing the first two generation vectors,
we got a lot of feedback that the titer of VSV-G peudotyped lentivirus produced
from these two sets of vectors were very low or even undetectable. We suspected
that the low virus titer production might be because of that the secondary
structure of IRES was perturbed by neighbor sequences such as cDNA insert. As a
result, the IRES loses their translation ability, so that virus transduced
cells were killed by drug due to no drug resistant proteins were synthesized.
We sought to solve this problem by replacing IRES by a human PGK promoter
(hPGKp) to direct the expression of selection marker. In addition, we also assessed
that the configuration of these promoter whether affect the the virus titer.
To compare the effects of IRES and/or promoter
position on virus titer, a panel of plasmids with puromycin marker was
constructed as shown in the following figure :
The pAS3w.puro is a second
generation vector from which the synthesis of PAC (puromycin resistant gene
product) is controlled by the IRES. As shown in above figure, the synthesis of
PAC in newly designed vectors (pLAS2w.Ppuro, pLAS3w.Ppuro, pLAS2w.puro, and
pLAS3w.puro) is controlled by an independent hPGK promoter. Among them, hPGK promoter cassette was placed
in the downstream of CMV or CAG promoter in pLAS2w.Ppuro and pLAS3w.Ppuro vectors;
while in pLAS2w.puro and pLAS3w.puro vectors, hPGK promoter cassette was placed
in the upstream of CMV or CAG promoter. “W” named in each vector represents the
WPRE sequence in which is positioned in the upstream of 3’-LTR (W does not show in the maps).
To compare the effects of insert length on virus titer in various
vectors , hepatitis C virus ORFs with different length, ranging from 0.8kb to
3.8kb (3.8kb insert is USP47, a gene
of deubiquitinating enzyme), were introduced into the downstream of CMV or CAG
promoter. VSV-G-peudotyped lentiviruses were produced from these resultant
vectors and transduced into A549 cells. The virus titers were analyzed by MTS
assay and represented as RIU/ul (relative infection unit per microliter). As
shown in the following table:
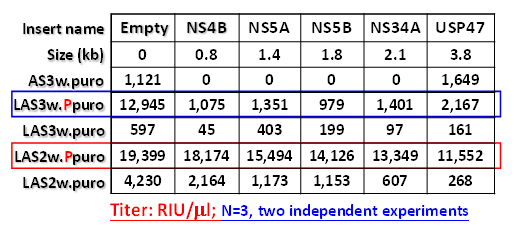
The results summarize as
follows: (i). The pLAS2w.Ppuro derived plasmids gave rise to the highest titer
compared to pLAS2w.puro and CAG-based series plasmids; (ii). The hPGKp
positioned in the downstream of CAG promoter is better than in the upstream of
CAG promoter in terms of virus production; (iii). The pLAS2w.Ppuro with 3.8kb
insert maintains high virus titer production, suggesting that the length of
insert can be longer than 3.8kb; (iv). The results indirectly support the
notion that IRES affects the translation of drug marker in some subtle way;
(v). The virus titer in pLAS3w.Ppuro series plasmids are low and the reason is
unknown. Although the length of CAG promoter is approximately 1.2kb longer than
CMV promoter, the difference of vector size in these two vectors may not
account for the virus titer difference. Because as compared pLAS2w.Ppuro with
2.1kb insert (NS34A) or with 3.8kb insert to pLAS3w.Ppuro with 0.8kb (NS4B)
insert, the virus titers of pLAS2w.Ppuro (with equal or longer size) are still
much higher than pLAS3w.Ppuro under the condition in terms of plasmid size.
However, we observed that the beta-actin promoter in CAG has a very high GC
content (above 74%), this high GC content might influence the process(s) of
virus life cycle such as virus assembly, reverse transcription. The affected
event(s) might lead to lower virus production. However, we do not have
experimental data to support this speculation.
To examine the promoter strength
of CMV, CAG, and TRE, luciferase ORF was introduced into pLAS2w, pLAS3w, and
pAS4.1w (inducible) series plasmids as indicated in the following figure:
The resultant plasmids were transiently tranfected into 293T cells and
luciferase activities were assayed at 48hrs post-transfection. As shown in above
figure, CAG promoter and TRE promoter had higher promoter activity than CMV
promoter. And promoter activity displayed some variations in vectors with the
same backbone but with different drug selection markers. Interestingly,
luciferase produced from pAS4.1w.Pbsd-aOn gave rise to the highest activity
(Pb.aOn). Interestingly, we observed that the spacer region between PAC-F2A-aOn and Bsd-F2A-aOn are different. Whether the sequences variation
affect the fusion protein processing is under investigation.
To further look into the luciferase
activity of those lentiviral transfer vectors in form of virus-transduced cells,
A549 cells were transduced with M.O.I. 0.1 to 0.2 VSV-G peusotyped lentiviruses
expressing luciferase as indicated to make sure that each cell receives
approximately 1 copy of luciferase gene. After selecting with corresponding
selection drug, resistant cells were expanded and seeded onto 96-well plate to
measure the luciferase activity. As shown in the following figure:
CAG promoter activity was higher than CMV
promoter activity which is consistent with transient transfection data in 293T
cells. The CMV promoter activity in different drug gene context also exhibited
some extent of variation. Notably, the luciferase activity was dropped quickly
from the first assay to the forth assay (approximately from 2 weeks to 5 weeks
after transduction). On the basis of this result, we suggest that one shall
freeze cells at very beginning for the future use.
In summary, the all-in-one inducible vectors are useful tool for the
studies of gene products with deleterious effects on cells, or the time and
space expression is in demand. For the constitutive expression vectors, we
provide a panel of vectors allowing you to choose depending on high virus titer
or expression level is required. In addition, these vectors provide as an
alternative tool to establish stable cell line rapidly.